Adapting to a New Lifestyle
In the midst of COVID-19 people have yearned for a saving grace, as the virus has led to a dramatic loss in human life worldwide and contributed to an unprecedented economic recession that challenges public resources, physical and mental health, food systems, and the world of work. Tens of millions of people are now faced with falling into extreme poverty, as well as millions of enterprises facing an existential threat. The workforce of the world is at risk of losing their livelihoods as the virus has made informal economy workers vulnerable to the loss of social protection and access to healthcare. COVID-19 has caused people to adjust to the new normal.

The daily lives of people around the world have been massively altered. Whether that be attending school and work virtually, carrying around a mask like it is as essential as a phone or wallet, or isolating from crowds larger than ten people, the population has settled into very different routines than they had in the past. In hopes that COVID-19 will become a routine of the past, the world has been awaiting the creation of a vaccine since the early days of the pandemic.
Vaccine Development
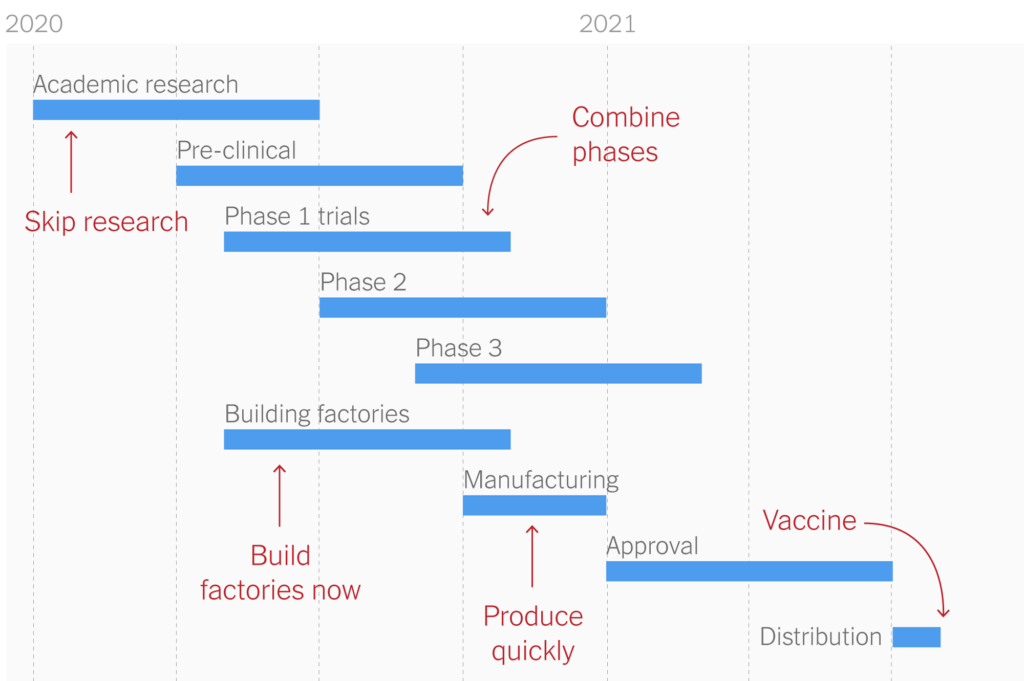
The goal of vaccines is to create herd immunity and develop sufficient protection in the community to prevent further transmission. Herd immunity is a concept that exists when enough people become immune to a disease, thus making the spread unlikely. Once an entire community is protected, those who are not immune are also themselves protected. This concept is usually only achieved through vaccination. Vaccine development is a long and grueling process. Vaccine development is broken into several stages, each one with a highly variable timeline. The fastest vaccine previously developed was for mumps, which took four years to develop. It typically takes around 10 to 15 years to develop a vaccine. After being produced, the vaccine is distributed to candidates in clinical trials. The experimental vaccine is given to small groups of people, and then grows to being distributed to larger and larger groups. This process can take years to complete. Following these trials, the vaccine has to face a regulatory review, in which the manufacturer submits an application for license to produce the vaccine. In the United States, this can take up to 10 months.
Vaccines work with the human body’s immune system to prepare it to combat potential infection. In the United States, there is currently no authorized or approved vaccine to prevent COVID-19.
Categorizing Vaccines
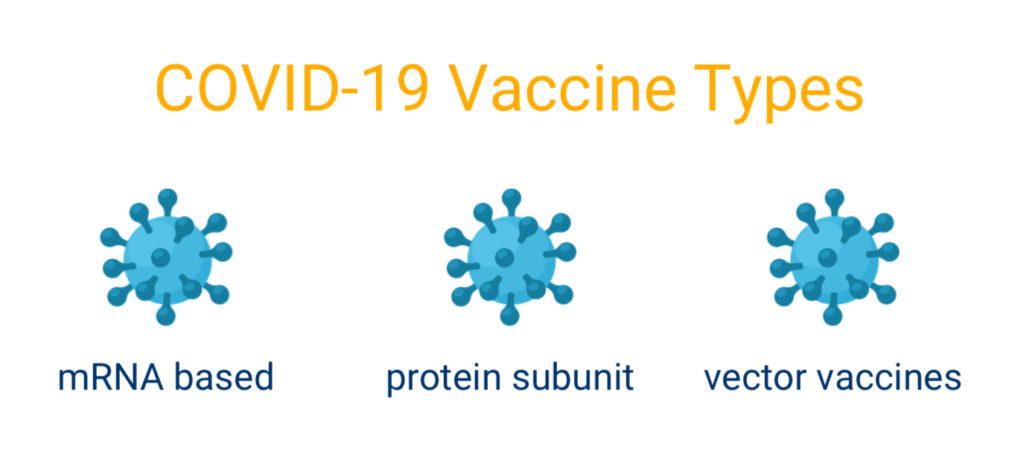
There are currently three main types of COVID-19 vaccines that are undergoing clinical trials in the United States; mRNA based, protein subunit, and vector vaccines. The first mRNA vaccines contain material from the virus that causes COVID-19, thus giving human’s cells instructions for how to make a harmless protein that is unique to the virus. Protein subunit vaccines include harmless pieces (proteins) of the virus that cause COVID-19 instead of the entire germ. Finally, vector vaccines contain a weakened version of the live virus, and is inserted in human cells to make a protein unique to the virus that causes COVID-19.
Two out of the three COVID-19 vaccines are in Phase 3 clinical trials in the US and will use two shots. The first will start building protection, while the second (injected a few weeks later) will provide the most protection that vaccines have to offer. The other vaccine in Phase 3 only requires one shot. The CDC has predicted that vaccines will begin to be available before the end of 2020, and will be accessible to the majority of adults later in 2021. Young children require further testing before they can be administered the vaccine.
“I’ve spent a career of over 35 years in vaccine development, and I can’t recall ever seeing a respiratory virus for which a vaccine provided this high a level of efficacy”
Bill Gruber, the head of vaccine development at Pfizer
An Tentatively Optimistic Future
On a Zoom call regarding the Pfizer-BionTech vaccine, the team learned that it was 95% effective. Their approach utilized the mRNA vaccine in which tiny fragments of the virus are used rather than dead or weakened versions. Pfizer has tested several different formulas for the vaccine but is still in the process of determining which is most effective. An additional hurdle to developing the vaccine is transporting it. Some of the vaccines require to be chilled to around -95 degrees Fahrenheit. If the FDA gives approval to the COVID-19 vaccines, they will soon be transported across the country in containers surrounded by super-frozen slabs. Moreover, another problem being addressed is making enough of the vaccine. Pharmaceutical companies like Pfizer and Moderna are making their vaccines in large tanks, 24 hours a day. Shipping carriers like FedEx and UPS have been preparing for these shipments by building freezer farms to ship large quantities of COVID-19 vaccines at extremely cold temperatures. The CDC plans on distributing the vaccine to health care workers and older Americans first, and hopes that by spring of 2021 the vaccine will be available to all who want it.
When it comes to the idea of this “saving grace” vaccine, the public needs to remain educated on the fact that social distancing should not cease once a vaccine is distributed. It is important to know that this shot does put an end to the pandemic, it is only the beginning of the end. There is not enough information regarding when people can stop wearing masks, or avoid close contact with others. This vaccine will not bring the COVID-19 pandemic to a halt, but it will assist in bringing the world closer to the finish line.
References
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
https://www.cbsnews.com/news/covid-19-vaccine-breakthroughs-distribution-cost-pandemic/
https://www.wired.com/story/why-creating-a-covid-19-vaccine-is-taking-so-long/
https://www.purdue.edu/caps/covid-19/adjusting-to-new-normal.html




